 |
| forest damaged by acid rain |
Acid precipitation is rain, snow, or mist which has a pH lower than unpolluted precipitation. Increased levels of acid precipitation have significant effects on food chains and ecosystems.
Precipitation—rain, snow, hail, sleet, or mist—is naturally acidified by carbonic acid (H2CO3). Carbon dioxide (CO2) in the atmosphere reacts with water molecules, lowering the pH of precipitation to 5.6. A pH scale is used to measure a solution’s acidity or alkalinity; pH is defined as the negative logarithm of the concentration of hydrogen ions, H+ . A solution with a pH of 7.0 is neutral. A pH lower than 7 is acidic, and a pH greater than 7 is alkaline.
Other acidic substances are also present in the atmosphere, causing "unpolluted" precipitation to have a pH approaching 5.0. Solutions with a pH of 5.0 or less have concentrations of hydroxyl ion, or OH– , and carbonate ion, or CO3– , approaching zero.
  |
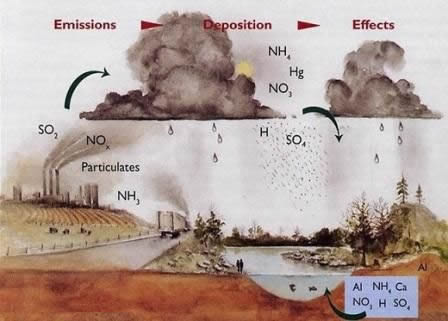
Acid precipitation is the name given to rain or snow contaminated with oxides of sulfur (SOx) and oxides of nitrogen (NOx). These chemicals combine with water droplets to form sulfuric acid and nitric acid. SOx is formed by combustion of materials containing sulfur, and NOx is formed by oxidation of molecular nitrogen in the atmosphere during combustion. SOx sometimes arises from natural sources such as volcanoes and geyser fields, and NOx is formed by lightning.
Downwind of smelting facilities, hydrochloric acid (HCl) and hydrofluoric acid (HF)may also contribute to acid precipitation. Acid precipitation may detrimentally change soil chemistry, either by stripping nutrients, especially magnesium and calcium, or mobilizing phytotoxic trace elements (elements toxic to plants).
Geographic Extent of Damage
Acid precipitation is a regional problem. SOx and NOx can travel many thousands of kilometers in the atmosphere after being emitted by large, stationary sources, especially those that have very high smoke stacks.
 |
| How acid rain is formed |
These pollutants are slowly transformed into sulfuric and nitric acid aerosols and are incorporated into precipitation, which eventually makes contact with the earth’s surface. Acid precipitation in the eastern United States contains more SOx than precipitation in the western United States, which contains more NOx.
In North America, acid precipitation and dry deposition (of acid aerosol particles) are major environmental problems in New England and New York State and in Ontario and Quebec. These regions attribute much of their acid precipitation to emissions from large coal-burning plants in the American Ohio Valley.
Scandinavian activists blame coal-burning power plants and factory emissions in the British Isles for that region’s acid rain problems. Central Europe—including Poland, the Czech Republic, Slovakia, and eastern Germany—has many power plants and factories that burn high-sulfur coal. Acid-laden pollution plumes stretch thousands of kilometers downwind from smokestacks in that region.
Controlled Studies
Controlled experiments on individual plant species have revealed short-term damage to a limited number of those species. Experiments using simulated acid rain (SAR) are difficult to extrapolate to field conditions, where the specific pollutants and pH levels vary widely over time.
In controlled conditions, studies showed no link between SAR and yield in Amsoy soybeans. However, field studies demonstrated that acid deposition does decrease yield in Amsoy soybeans.
 |
| Air Pollution |
Acid precipitation influences plant diseases by acting on both pathogens and host organisms. Seedlings of Pinus rigida, Pinus echinata, Pinus taeda, and Pinus strobus exposed to SAR of pH 3.0 had a 100 percent mortality rate because of fungal damping-off, a diseased condition of seedlings marked by wilting or rotting.
Red spruce seedlings subjected to dilute sulfuric acid mist developed brown lesions on their needles, followed by needle drop. Studies showed a reduction in the growth of sugar maple seedlings following exposure to low pH moisture, and that seedling survival decreased with increasing acidity.
Crop and Forest Decline
In field experiments, soybeans have shown reduced yields with decreasing pH (increasing acidity) of moisture applied. Yields of seed and seed protein are both reduced in soybeans exposed to high acidity. A lower number of seed pods were found in plants exposed to high acidity, compared to control plants.
Acid precipitation causes detrimental long-term effects in most ecosystems, especially forests. Root systems under acidic stress show great variability in tolerance and injury. Acidic stress on roots decreases root growth, measured by a reduction in root length, and severely damaged trees have more fine roots with opaque tip zones than do slightly damaged trees. Some scientists have suggested that the radical growth rate in yellow pines in the southeastern United States may be reduced by acid precipitation.
Since the 1960’s Central European soils have been progressively acidified, altering soil buffering capacities. Acid rain containing nitrates (which are not immobilized in soil) played an important role in this soil acidification.
Acidification has reduced the magnesium, calcium, and potassium available for nutrient uptake by plants and has affected root growth. One-quarter of European forests are moderately or severely damaged by acid precipitation, with dry deposition believed (by scientists and politicians) to be largely responsible.
This pattern of damage, first detected in the 1980’s, has been called neuartige Waldschäden (literally, "new-type forest decline"). It has been detected throughout Central Europe at all elevations and on all soil types. Waldschäden is most pronounced downwind of major air pollution sources.
Abnormally high numbers of red spruce have died in the high-elevation northern Appalachian Mountains since the 1960’s. This die-off has been attributed to high rates of acid deposition (up to 4 kilo equivalents of hydronium ions per hectare per year) and exposure to acid fog droplets for up to two thousand hours per year. Very high levels of trace metals (known to be phytotoxic) have accumulated in the region.
Aquatic Ecosystems
Most freshwater ecosystems range from pH 6.0 to pH 8.0. In limestone terrain, acid precipitation is neutralized by dissolution of calcium carbonate. As a freshwater environment becomes acidified, the number of species it supports declines. When conditions are more acidic than pH5.5, dissolved inorganic carbon exists only as dissolved carbon dioxide. Planktonic algae, which can use low levels of dissolved inorganic carbon, are favored in these environments.
Acid environments greatly reduce the numbers of herbivores that graze on aquatic plants; this is thought to explain why filamentous green algae are found in most acidified lakes. Scientists point out that it is difficult to separate the effects that acidification alone produces in an aquatic ecosystem.